I have to start with a confession: I’ve felt a pretty significant degree of frustration discussing women’s health in the Indian ecosystem. Too often, it’s treated as an afterthought. Take the case of menstrual health, which is wrongly seen as a hygiene issue but not a healthcare issue. Given this starter bias and also historical imbalance in womens’ health research, the realm of investment scope gets limited to things like pads and menstrual cups. Even basic issues like Dysmenorrhea or IBS conditions that can cascade into serious complications are brushed aside. Our medical cannabis origins make conversations even harder to take towards a genuine meaningful conversation. I won’t lie, this may be the actual problem, which makes things a bit more sad.
Meanwhile, Israel and China are moving fast with a solving mindset pushing cannabinoid/polyherbal science (Yes, China too! where they may otherwise hang you for possession), running pragmatic trials, and evolving regulatory pathways. Western companies have even built entire models around “catching early Phase-I innovations in China” and purchasing rights for the West later. It’s smart and now crossed billions of dollars in value. Why, then, do we in India sitting at ground zero of mass ailments, struggle with impostor syndrome?
Silicon Valley is often accused of “privileged innovation,” and sure, it builds for affluent markets. But reminds me of the Jeff Bezos rule of unchangeables that applies almost everywhere: nobody wants things slower; nobody wants things more expensive. India can build better and more affordable health solutions precisely because we’re closest to the real-world burden.
I’m Indian and a father of two daughters. This is personal. After years of hitting walls trying to convey the long-term human and economic costs of ignoring these “everyday” ailments, I’ve decided to publish what we’ve learned, share our data, and invite anyone serious about solving this to work with us, wherever in the world you may be. We are currently supported by our pack of strays who have gotten us this far and we ain’t stopping.
The Hidden Burden (and Why This Should Be a Global Priority)
Prevalence :
- Dysmenorrhea (menstrual pain) affects ~50–90% of post-pubertal women (definitions vary by severity). Presenteeism and absenteeism hit students and workers hardest.
- IBS affects ~4–10% of adults globally (10–15% in some U.S. estimates), with women disproportionately impacted; IBS-D is ~one-third of cases.
Productivity and economic costs
- Menstruation-related symptoms drive large productivity losses; one large study found ~8.9 days/year lost and ~33% presenteeism during symptomatic periods.
- In Australia, women with chronic pelvic pain (including endometriosis/dysmenorrhea) face Int’l $16,970–$20,898 per woman/year, 75–84% due to productivity loss.
- IBS carries heavy direct medical costs; U.S. estimates put annual direct costs ~US$6,182 per patient vs US$4,156 controls (2013 USD); additional studies show higher totals for subtypes and payer cohorts.
- IBS also crushes on the job performance, older employee studies show ~15–21% productivity loss vs non-IBS peers.
Policy context
This burden touches multiple SDGs - SDG 6.2 (sanitation/hygiene, calling out needs of women and girls), SDG 3.7 (sexual & reproductive health services), SDG 5.6 (reproductive health/rights) even though “menstrual health” isn’t a standalone SDG indicator.
The funding tide is turning
In August 2025 the Gates Foundation committed US$2.5B through 2030 to women’s health R&D, explicitly including gynecological and menstrual health—its largest such allocation ever. Major outlets and the Foundation detail focus areas and the historic research gap.
Off-Label and Self-Medication: Real Harms We Can Measure
Because condition-specific therapies and trials rarely exist, many patients default to NSAIDs for dysmenorrhea and acid-suppressants/PPIs or H2 blockers for gut symptoms, often for years.
- NSAIDs: The FDA flags community-acquired acute kidney injury precipitation risk from NSAIDs; chronic use increases risk of CKD, GI ulcer/bleeding, and liver toxicity.
- PPIs (for “IBS-adjacent” reflux/dyspepsia self-treatment): Systematic analyses associate long-term PPI use with CKD/AKI and nutrient malabsorption (B12, iron, Mg, Ca), increasing risks of anemia and fractures.
- NSAID+PPI paradox: While PPIs can protect the upper GI tract, recent large cohort work shows higher lower-GI bleeding risk with NSAID+PPI vs NSAID alone (HR ~2.84). ([PMC]
- NSAIDs for IBS pain? Evidence reviews do not recommend NSAIDs for IBS pain control (and they may worsen gut side-effects).
Bottom line: off-label isn’t benign. It’s a slow-motion pipeline into kidney disease, GI bleeding, anemia/osteoporosis, and chronic pain that silently inflates future healthcare costs.
What Happens When Dysmenorrhea/IBS Are Poorly Managed or Ignored
Dysmenorrhea (and related gynecologic conditions)
- Endometriosis progression → adhesions, chronic pelvic pain, infertility; the national burden runs into $7.4–$9.7B/year in Australia alone.
- Adenomyosis & fibroids → heavier bleeding, worsening pain, surgical risks if unaddressed.
- Chronic pain sensitization → repeated unmanaged pain “rewires” pain pathways.
- Mental health → higher depression/anxiety and education/work disruption
IBS (when unmanaged or mis-managed)
- Productivity: persistent ~15–21% loss in work productivity; presenteeism dominates. ([PubMed]
- Comorbidity costs: comorbid depression/anxiety dramatically raises total costs (e.g., +$6,709 incremental direct costs with comorbid depression in one claims analysis). ([Value in Health]
- Medication harms: chronic NSAIDs/PPIs add renal, GI, hematologic, and skeletal risks. ([U.S. Food and Drug Administration]
Polyherbal Solutions: The Missing Middle (and Why India Should Lead)
The modern regulatory/scientific machine was built around single molecules, great for acute/lethal diseases, but a poor fit for multi-component, non-fatal, high-prevalence conditions like dysmenorrhea and IBS. Result: polyherbal products are widely used but rarely trialed, so the world leans on off-label stop-gaps.
- Israel has long led cannabinoid and plant-based research (from Mechoulam’s foundational work onward) and built supportive infrastructure for medical cannabis as an instructive template for polyherbal progress.
- China has been reforming TCM regulation, approving new TCMs under evidence frameworks and pushing for multi-center, higher-quality trials explicitly tackling the evaluation gap for multi-component medicines.
Why this matters: Polyherbal formulations encode centuries of practice but face combinatorial complexity (dozens of actives; many targets). Traditional trial design treats this as “too messy.” That’s precisely where AI + Quantum excel.
Our Trial: What We Ran, Why We Restarted, and What We Learned
At HempStreet, we developed #FormulationFemme, a polyherbal microdosed cannabis-based formulation for dysmenorrhea, and conducted what we believe was the world’s first dysmenorrhea Phase-I clinical trial. It was rigorous, ethics-committee guided, and restarted once to ensure the best representation of both the ailment and the solution (i.e., tighter inclusion/exclusion and a formulation/endpoint setup reflecting real-world patients). Also, does feel pretty cool that this little scrappy company out of India led the world into this direction ![]()
Key takeaways:
- Phase I is non-negotiable for mass ailments: human safety under ethics oversight is essential when use is chronic/recurrent.
- Off-label is not a strategy: one-size-fits-all NSAIDs/PPIs aren’t optimized for menstrual or IBS biology and may carry long-term risks.
- Cost/design are the chokepoints: under a single-molecule paradigm, Phase II/III for polyherbals is too complicated, expensive and too slow for most sponsors.
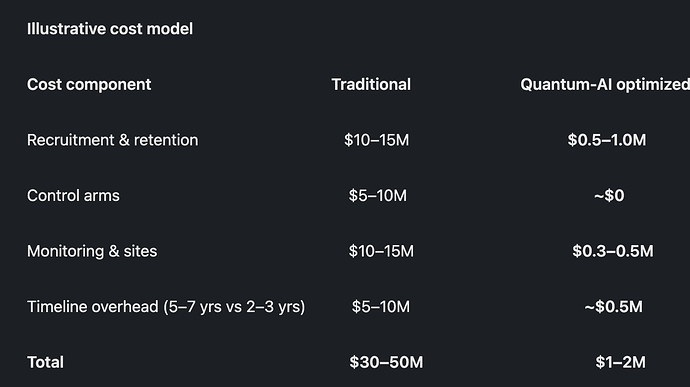
How Quantum-AI Makes Phase II/III Affordable (US$1–2M Range)
The playbook is to combine AI digital twins, synthetic/external controls, and quantum optimization for design choices:
- Shrink enrollment with digital twins: simulate large, diverse populations; run 50–100 real participants for validation instead of thousands.
- Ditch full control arms: use synthetic/external controls from real-world data where appropriate, already recognized in limited regulatory contexts.
- Adaptive platforms: quantum optimizers help tune dose, subgrouping, and site selection in near-real-time to cut waste.
- Virtualized execution: e-consent, remote monitoring, wearable endpoints → fewer sites, lower monitoring costs.
- Smarter endpoints: early biomarkers and digital signals for faster, smaller trials.
Illustrative cost model
Who’s building the stack: SandboxAQ (AI+quantum platforms), isomorphic labs (DL for drug design), quantinuum (quantum chemistry/ML), Rigetti (optimization) and others are laying the rails for this hybrid future.
The Research Gap Is Real (and Funders Are Responding)
Women-specific conditions (beyond cancer) have historically received ~1% of pharma research funding, a failure now being addressed by new commitments like Gates’ US$2.5B (40+ targeted innovations across maternal, gynecologic/menstrual health, contraceptives, STIs).
This creates an opportunity to prioritize polyherbal, non-fatal ailments that drive massive hidden costs through presenteeism, absenteeism, and downstream comorbidities.
The Zero-to-One Opportunity (and a Call to Collaborate)
Imagine a world where:
- Women aren’t left on NSAIDs/PPIs for years with growing renal/GI/bone risks.
- Dysmenorrhea and IBS have trialed, labeled, polyherbal options, validated via Quantum-AI and RWD.
- India leans into its scale and know-how to deliver global-first innovation - faster, better, more affordable
We’re ready to share all the years of data from our Phase-I program and the operational lessons we’ve learned.
Final Thought
Israel and China have shown this can be done. Western firms already buy early rights from those ecosystems. Silicon Valley may innovate for privileged markets, but it does innovate. India sits on the world’s densest burden of mass ailments. We have the patients, clinicians, traditional knowledge, data, and entrepreneurs. What we need now is the mindset.
Bezos’ rule stands: no one wants slower or more expensive. With AI + Quantum, polyherbal trials become faster and cheaper and we can make them better, too. With major funders stepping up, the path from improvisation to validation is open. We’ve reached out to all the western folks but would love to also work with any folks in the space from India.
I write this as an entrepreneur and as a father of two daughters. This matters to me deeply. It should matter to all of us.